✍️ Author: Dr Eleni Christoforidou
Home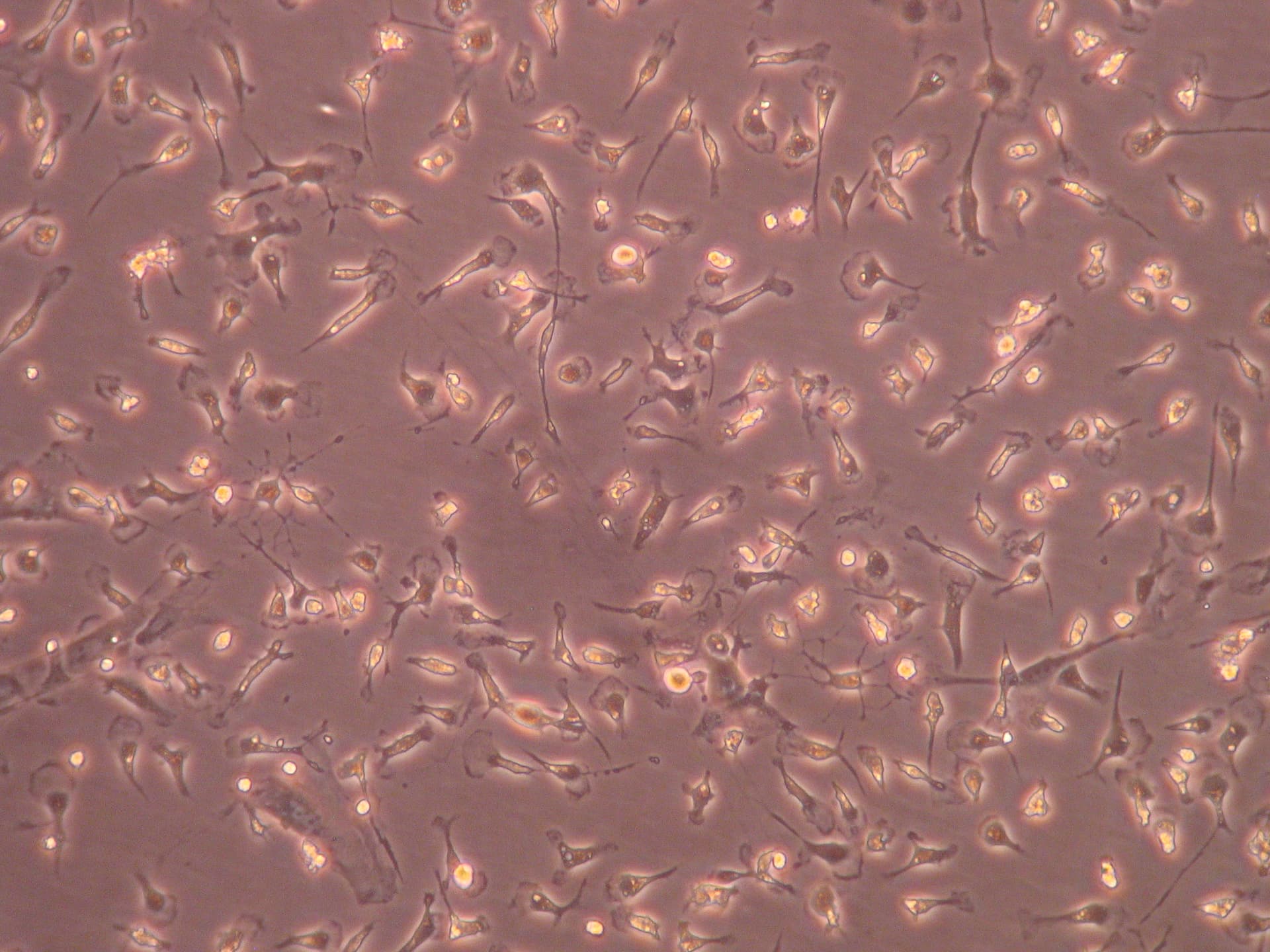
Intricately cultured microglia, extracted from a mixed glia culture of cells originally dissected out of neonatal brains.
A Day in the Lab: Investigating Isolated Microglia from Neonatal Brains
🕒 Approximate reading time: 3 minutes
Today's exploration in the lab led me into the intricate realm of brain microglia. These cells were selectively isolated from a mixed culture containing both microglia and astrocytes, a task accomplished three days prior. These microglia, originally dissected from neonatal brains, were then cultured in a flask, priming them for today's investigation.
To examine them, I utilised a phase-contrast microscope, offering me a clear and detailed perspective of their microscopic world. I was particularly interested in analysing the morphology of the microglia and the purity of the culture, essential factors to confirm the success of the isolation experiment. The image below unveils the captivating sight of the cells I inspected today.

Diving Deeper into Today's Experimental Approach
The isolated microglia were cultivated in the lab using a gentle trypsinisation method. Initially, the culture medium was withdrawn, and the cells were rinsed with Dulbecco's Phosphate-Buffered Saline (DPBS). The mixed glia-conditioned medium underwent a 0.2 μm filter treatment to eliminate any cell debris.
The cells were then treated with trypsin-EDTA, diluted 1:4 in Dulbecco’s Modified Eagle’s Medium (DMEM), and incubated under specific conditions (37°C, 5% CO2) for 45 minutes. This step served to detach the astrocyte layer, and the trypsin activity was subsequently halted by adding culture media.
Following this, the medium was fully aspirated to discard the detached astrocytes and oligodendrocytes, and the cells were thoroughly washed with DPBS. The cells left behind were the adhered microglia, which were then nurtured in 50% mixed glia-conditioned culture medium under optimal conditions (37°C, 5% CO2) for two days until they resumed their ramified form.