✍️ Author: Dr Eleni Christoforidou
Home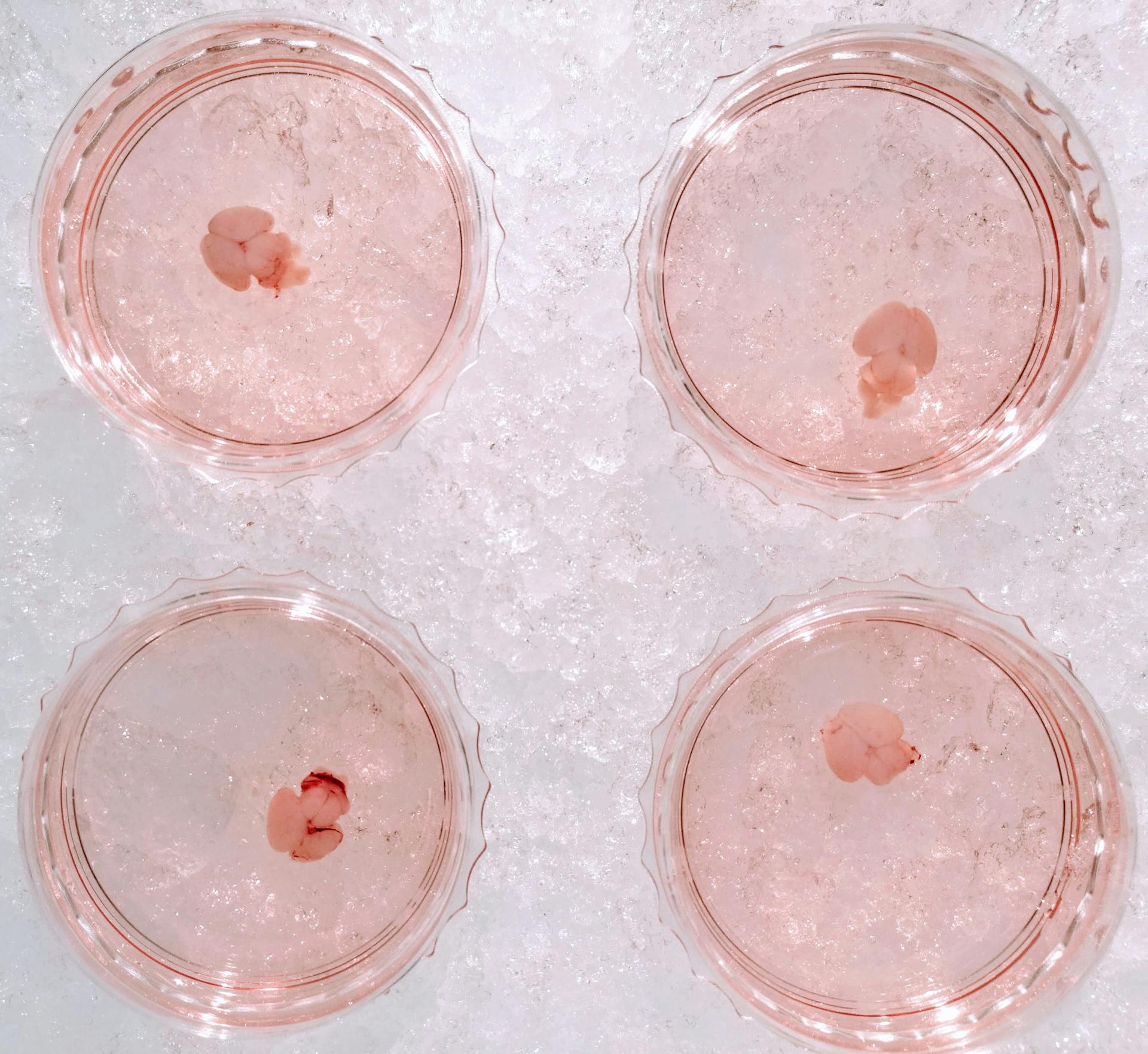
Neonatal brains meticulously dissected for today's riveting experiment.
A Day in the Lab: Delving into Neonatal Brain Dissection for Glial Cells
🕒 Approximate reading time: 3 minutes
In the lab today, I embarked on the meticulous task of dissecting neonatal brains. The goal was to extract glial cells and culture them for my ongoing experiments. The task was time-intensive; dissecting a total of seven brains occupied roughly 6.5 hours of my day, all without pause. The procedure itself is reasonably straightforward, albeit requiring exceptional dexterity given the delicate and diminutive nature of the neonatal brains.

Delving Deeper into Today's Experimental Methodology
From each brain, the hippocampi and cortices were diligently dissected and minced using spring scissors. The tissue harvested from each brain was then transferred into a cold dissection medium, ready for the addition of trypsin to dissociate the cells.
Following this, the cells were incubated in a 37°C water bath for 15 minutes, ensuring frequent swirling. Next, a trypsin inhibitor was introduced to halt trypsin activity, with the tube left to incubate for a brief period (1 minute) at room temperature.
Subsequently, DNase I was added to the mix to digest the sticky DNA released from the dead cells, followed by centrifugation of the tube at 400 × g for 5 minutes. Once the supernatant was removed, the pellet was triturated in pre-warmed culture medium, then subjected to another round of centrifugation at the same speed.
Finally, after the supernatant's removal, the pellet was resuspended in 5 ml of pre-warmed culture medium. This resulting cell suspension was then cultured in a PDL-coated T-75 tissue culture flask, again with pre-warmed culture medium, and incubated under specific conditions (37°C, 5% CO2).